Does water conduct electricity ? and why pure water doesn't conduct electricity and normal water does ?
Yes, water may or may not conduct electricity depend upon whether the water is pure or impure .
- Pure or distilled water contains no ions as it does not form ions due to dilution of pure water with acids, bases or salts. But tap water contains many dissolved salts that form ions in the solution. Therefore, tap water conducts electricity, but distilled water does not.
- Any impurities such as salt in water will make it conductive. When salts dissolve in water, they break up into different electrically charged particles called ions.
- Salt or sodium chloride (NaCl) dissociates into positive Na ions and negative Cl ions. If you put the negative battery in water, negative ions will act on the positive terminal and positive ions will act on the negative terminal
Why pure water doesn't conduct electricity ?
- In order for electric current to pass through a liquid, charges must move through the liquid. In case of distilled water All of the water is deionized that is "pure" water without any ions. Therefore, pure water does not conduct electricity because there is no charge in the water.
- Distilled water has no impurities and therefore no ions. There are only intermediate molecules, and these intermediate molecules have no value. Therefore, distilled water doesn't have capability to conduct electricity
Why Normal water conduct electricity
Normal water like Tap water sea water includes impurities, such as dissolved sodium, calcium, and magnesium salts, these impurities are good conductor of electricity because of these impurities electric current flow through normal water or impure water.
Why does sea water conduct electricity and and solid Salt doesn't
- Ions present In solid Salt is immobile . When the solid salt dissolved in water the immobile ions become mobile and can carry an electric current.
- In water, ionic compounds like salt split into postive and negative charged ions and are attracted to the electrode with opposite charges.
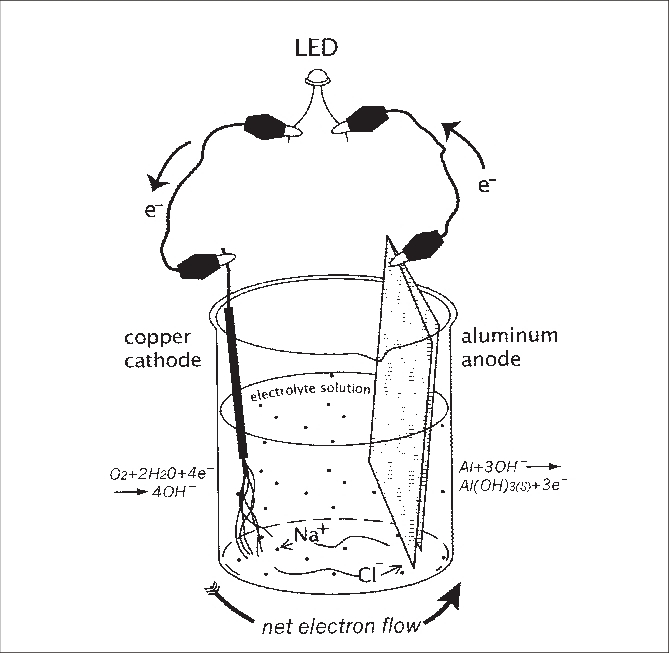
Is salt water or sea water can light up the bulb ?
- Yes salt water or sea water light up the bulb In this experiment you should have a simple circuit consisting of a light bulb, two metal electrodes, and a voltage source (A battery). When you connect the two electrodes to the battery, one electrode becomes positively charged (anode), and the other becomes negatively charged (cathode).
- The positively charged sodium ions (Na+) are attracted to the negatively charged electrode (cathode), while the negatively charged chloride ions (Cl-) are attracted to the positively charged electrode (anode). This movement of ions toward the respective electrodes allows for the flow of electrical current through the saltwater. Thus light up the bulb.